Laser Processing of Flexible In-Plane Micro-supercapacitors: Progresses in Advanced Manufacturing of Nanostructured Electrodes | ACS Nano
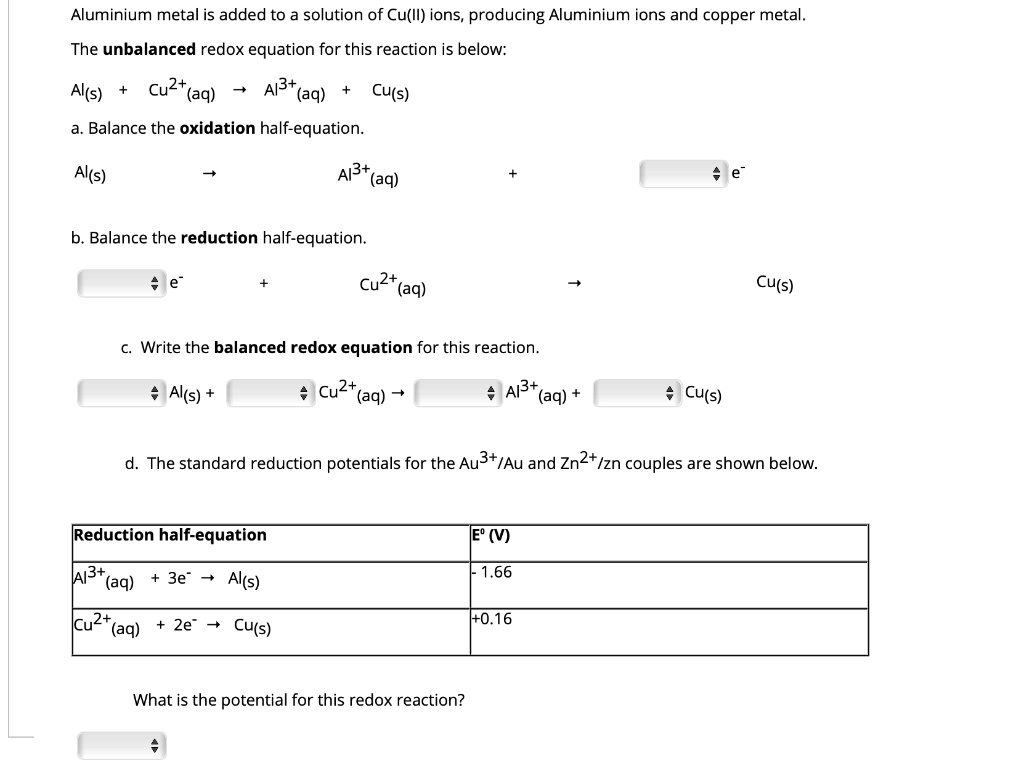
SOLVED: Aluminium metal is added to a solution of Cu(Il) ions, producing Aluminium ions and copper metal. The unbalanced redox equation for this reaction is below: a. Balance the oxidation half-equation. Al(s)

Spreading the Landscape of Dual Ion Batteries: from Electrode to Electrolyte - Liu - 2023 - ChemSusChem - Wiley Online Library

Synthesis of Alum Lab This synthesis reaction involves a redox reaction and the formation of a complex ion. - ppt download

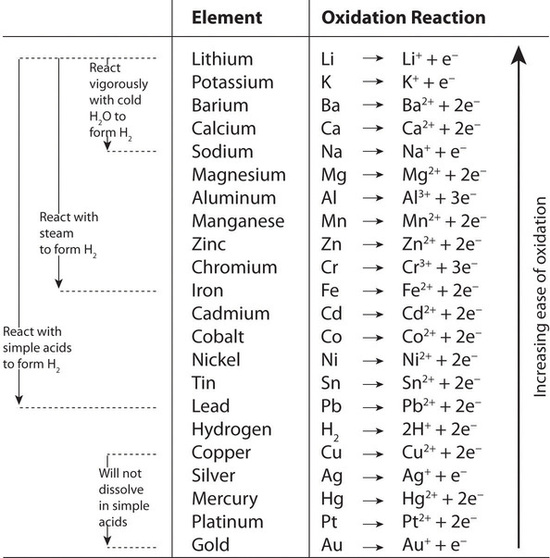
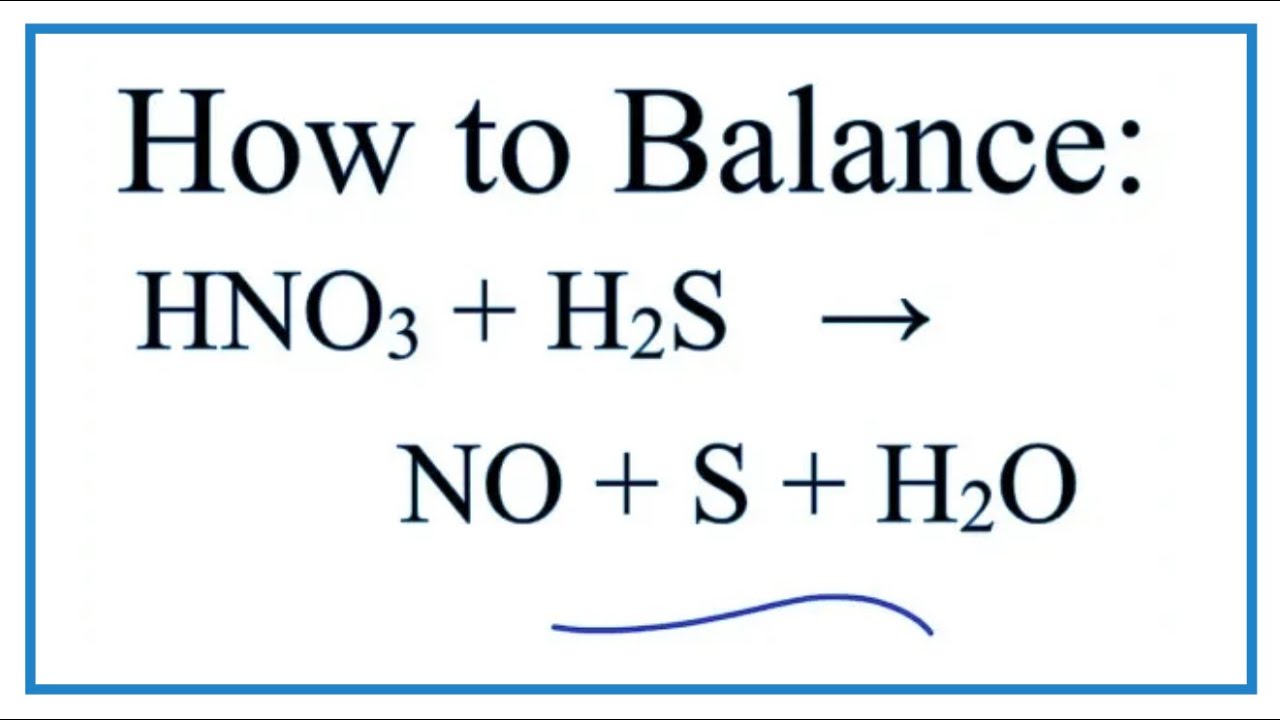
Redox Difficult but necessary. Obviously: Oxidation is adding oxygen 2H 2 + O 2 2H 2 O Reduction is removing oxygen 2FeO + C 2Fe + CO 2 But also oxidation. - ppt download

Chemistry 12 Unit 5. I.Oxidation – Reduction Reactions: Oxidation:A substance losing electrons. Reduction:A substance gaining electrons. eg:Al (s) in. - ppt download

Recent Advances in Organic Reactions Involving Elemental Sulfur - Nguyen - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library

Calculate the number of aluminium ions present in 0.051 g of aluminium oxide. (Hint: The mass of... - YouTube

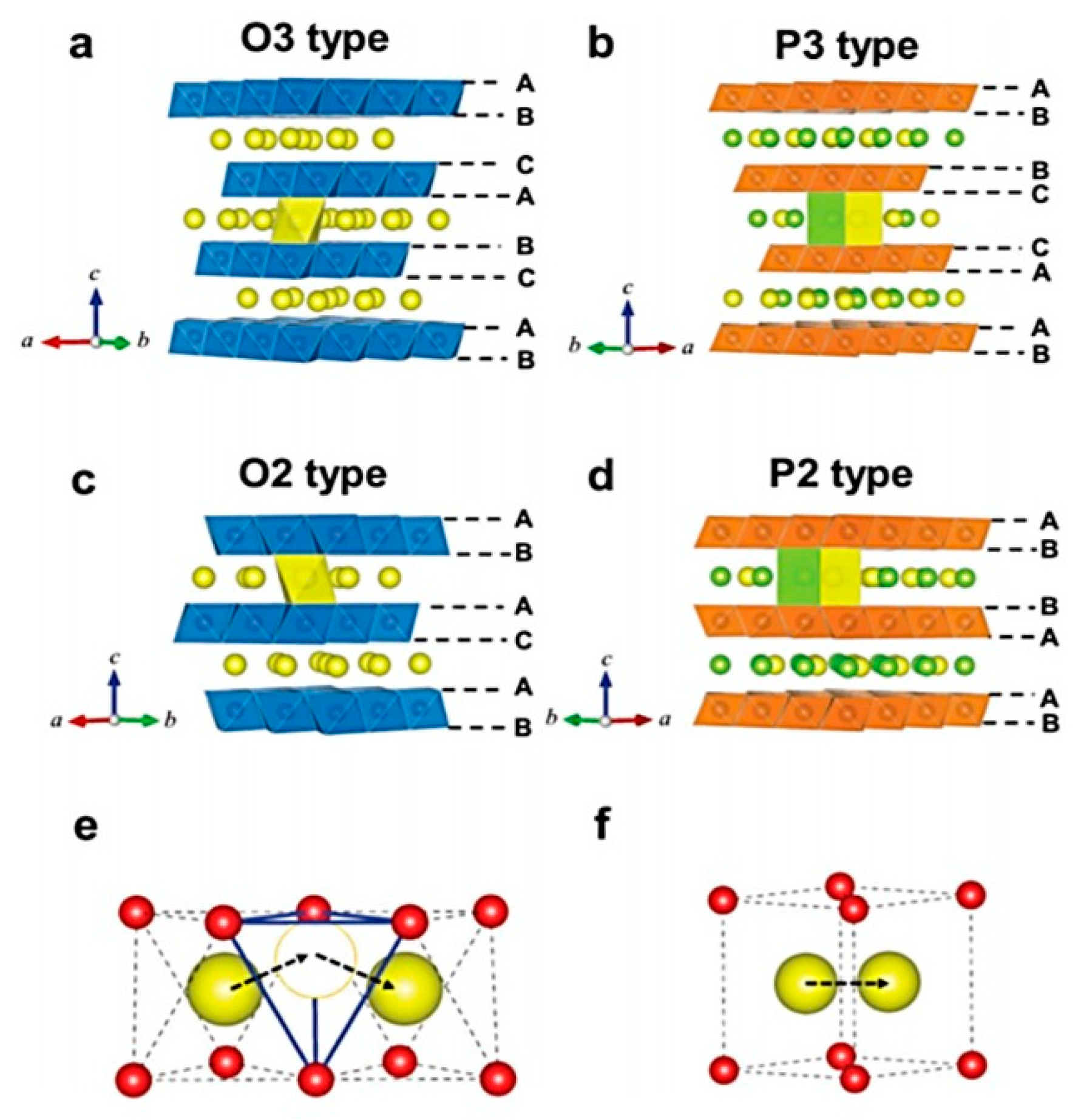
Crystallographically Textured Electrodes for Rechargeable Batteries: Symmetry, Fabrication, and Characterization | Chemical Reviews