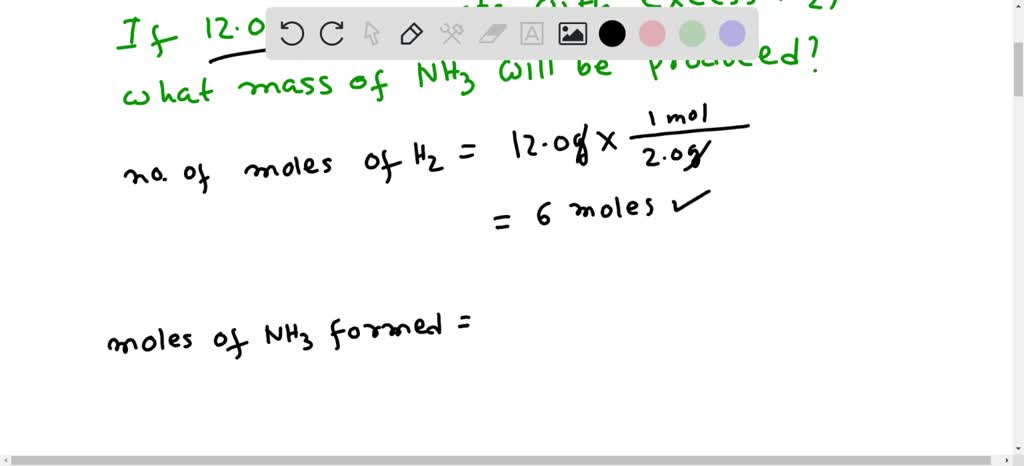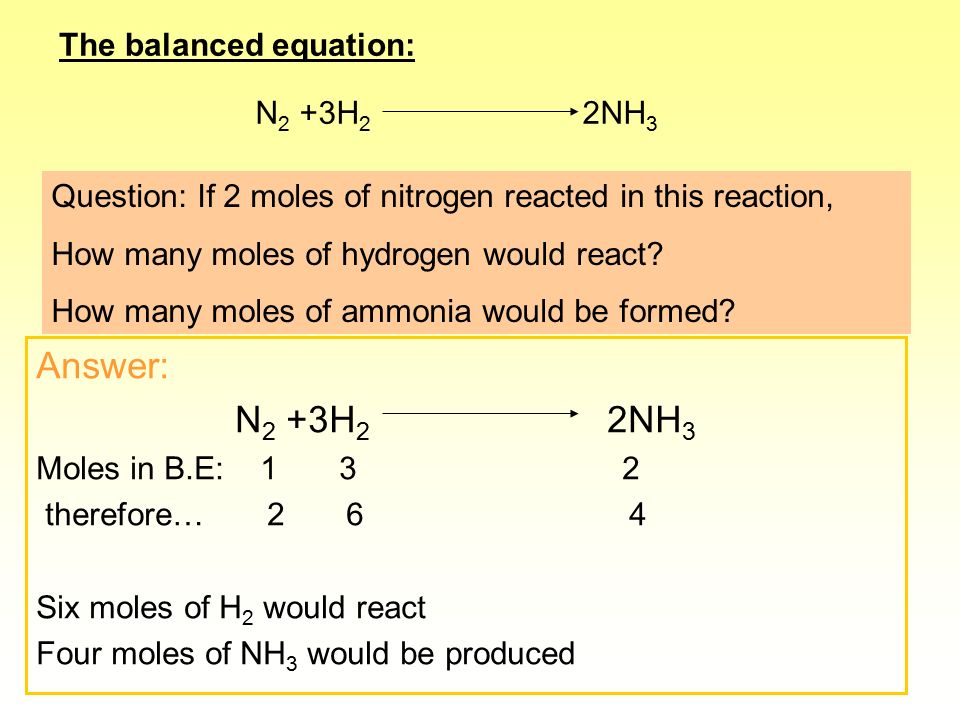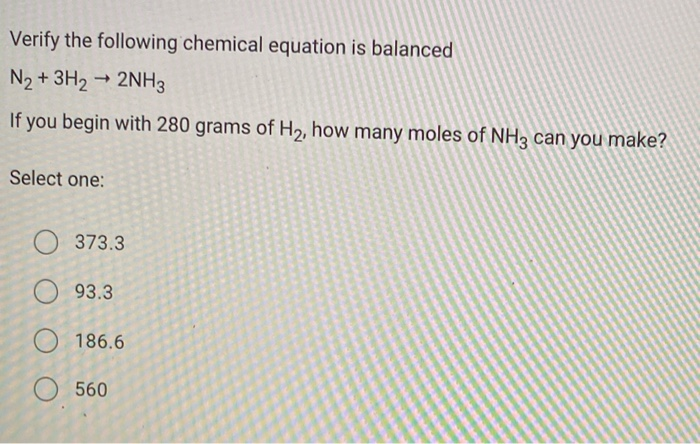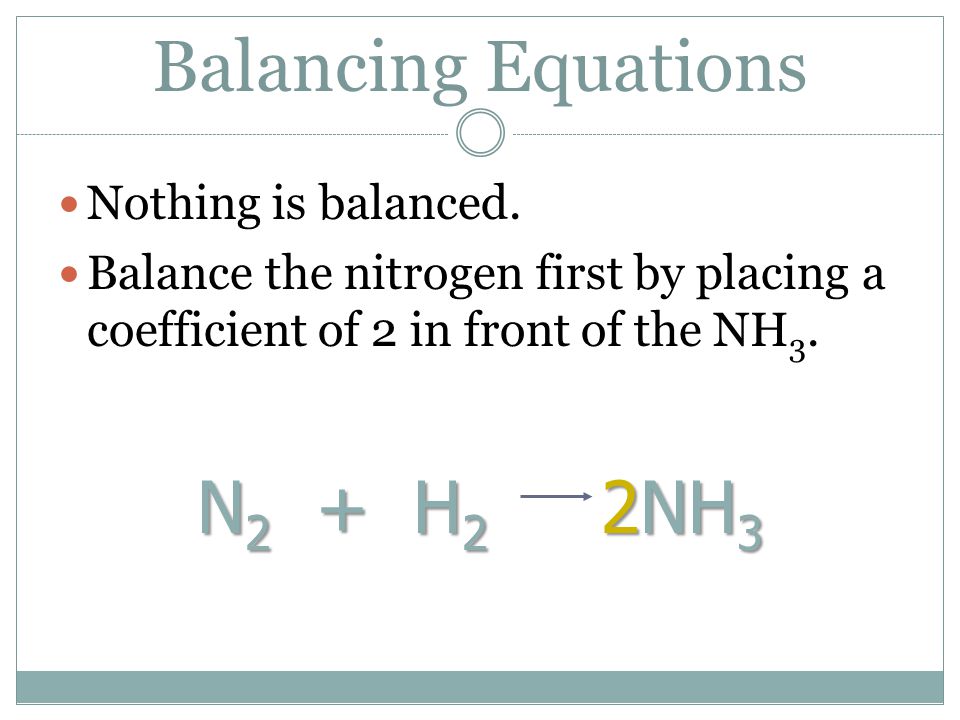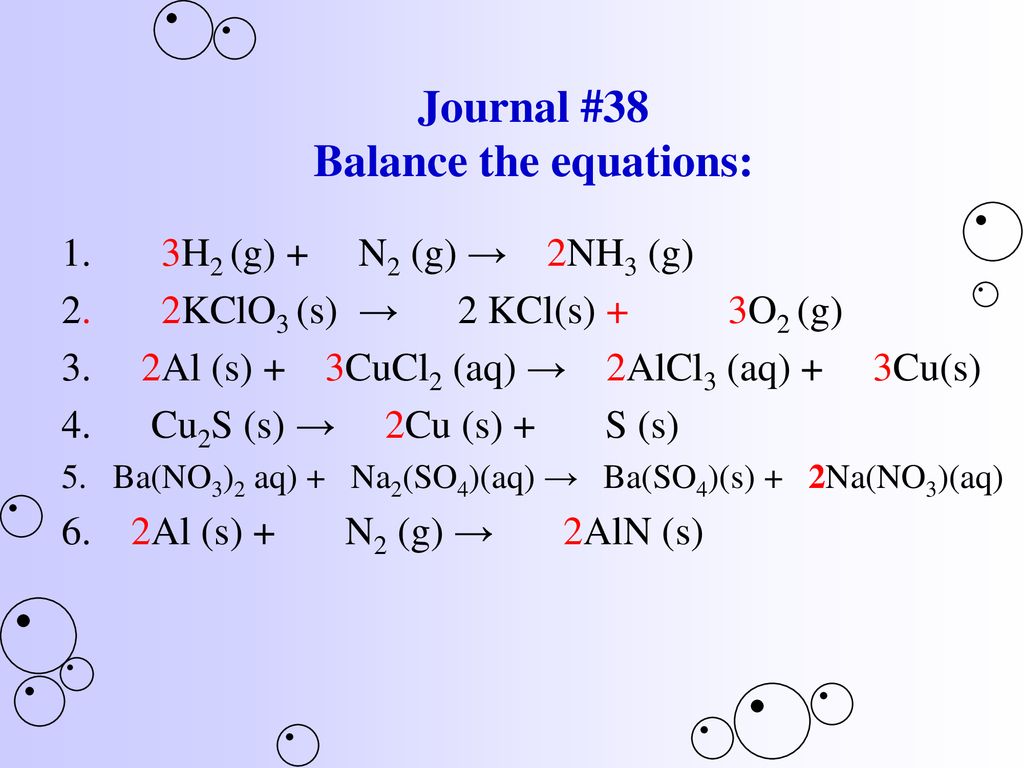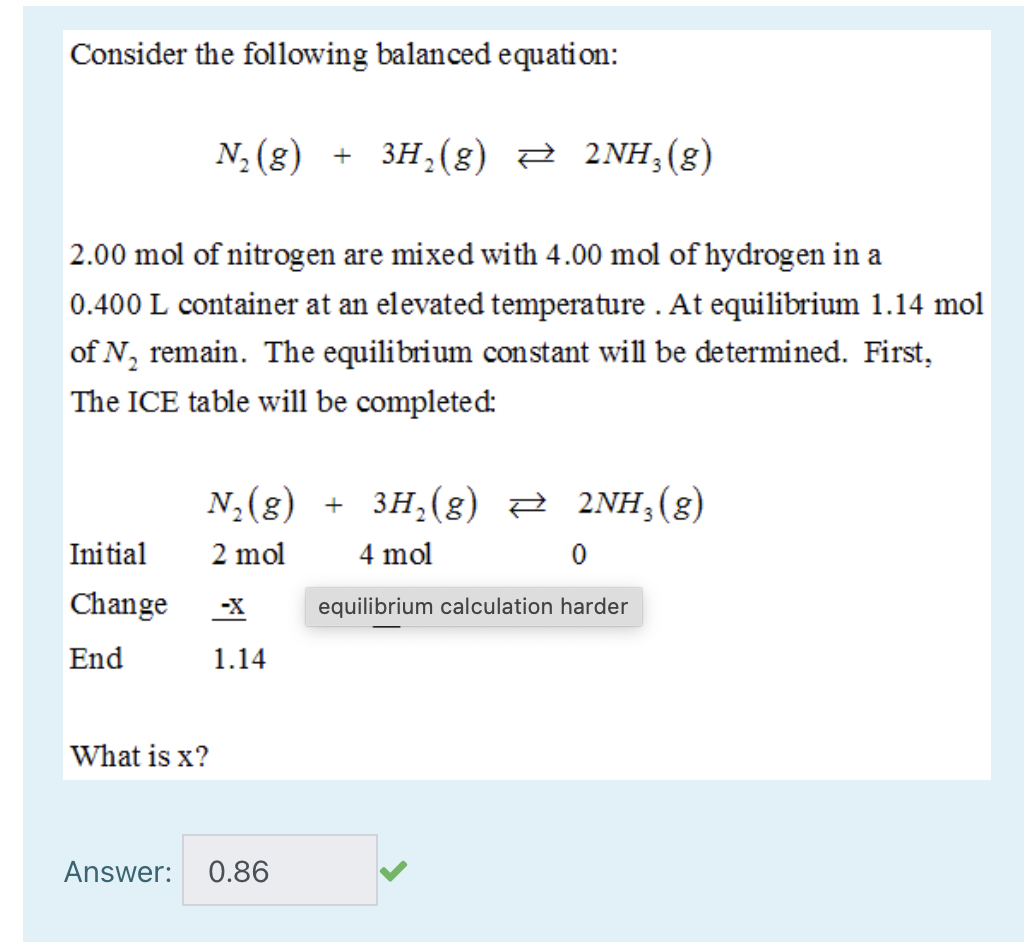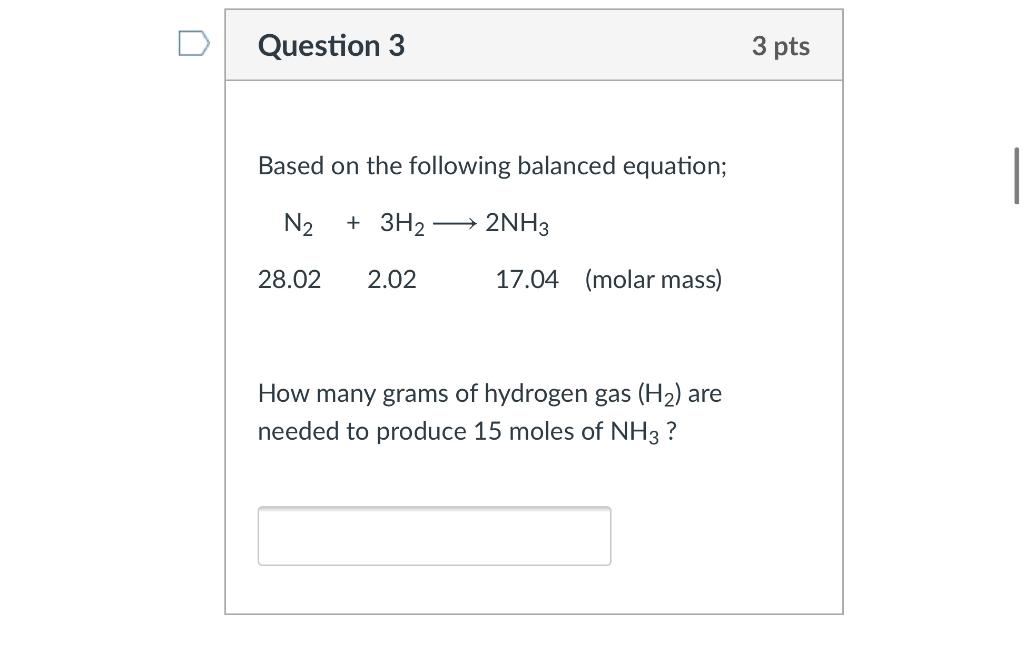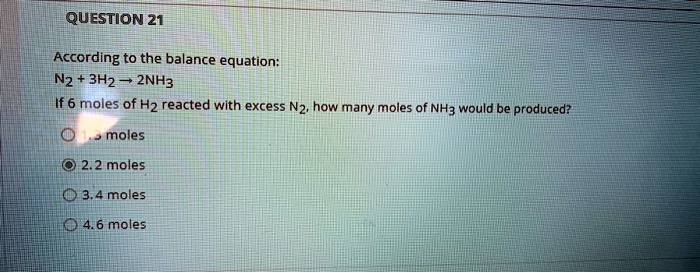
SOLVED: QUESTION 21 According to the balance equation: N2 3H2 2NH3 If 6 moles of Hz reacted with excess Nz, how many moles of NHa would be produced? moles 2.2 moles 03.4 moles 014.6 moles

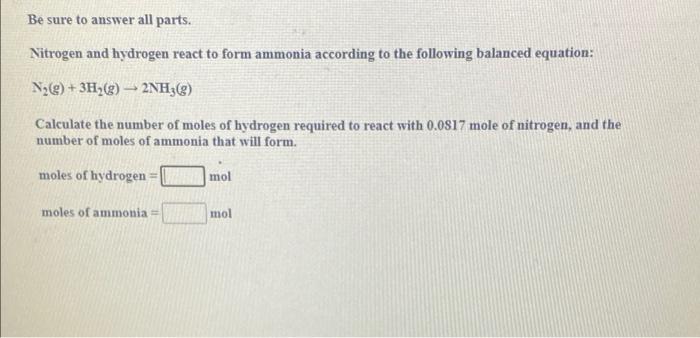
Bell Ringer Jan. 31 N2 + 3H2 2NH3 How many moles of NH3 are created from 18 grams of H2? How many moles of N2 are needed to create ppt download

For the reaction, N2 + 3H2→ 2NH3 . The rate of change of concentration for hydrogen is 0.3 × 10^-4Ms^-1 . The rate of change of concentration ammonia is:

N2 +H2 =NH3 Balanced Equation - n2 + h2 → nh3 balance||Nitrogen+Hydrogen=Ammonia Balanced Equation - YouTube